Yes, propane is indeed a chemical. To clarify, a chemical is any substance consisting of matter. This includes any liquid, solid, or gas that is composed of molecules or atoms. Propane fits this definition as it is a hydrocarbon with a specific molecular composition of three carbon atoms and eight hydrogen atoms (C3H8).
It is a volatile, flammable gas that occurs in natural gas and crude oil and is commonly used as a fuel. The properties and behaviors of propane, such as its energy content, flammability, and how it reacts with other substances, are determined by its chemical structure.
Chemical Structure and Composition of Propane
A. Molecular Formula and Atomic Composition
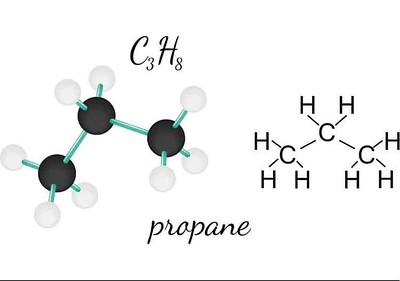
Propane’s molecular formula is C3H8, indicating it contains three carbon atoms and eight hydrogen atoms. Each carbon atom is bonded to the other carbons and to hydrogen atoms in a specific arrangement that defines its chemical structure.
B. Bonding and Arrangement of Atoms in a Propane Molecule
In a propane molecule, the three carbon atoms form a chain with single covalent bonds between them. Each carbon atom also forms single covalent bonds with hydrogen atoms, fulfilling the carbon’s valency of four. The central carbon atom is bonded to two hydrogen atoms and the two end carbon atoms are each bonded to three hydrogen atoms.
C. Isomerism and Structural Variations in Propane Derivatives
Propane itself does not exhibit isomerism due to its simple structure. However, propane derivatives can display structural variations. For example, if a hydrogen atom is replaced by another atom or group of atoms, the resulting compound is a propane derivative, which might show isomerism based on the position of the substitution.
How do you classify propane?
Propane is classified in several ways based on its chemical structure, physical state, and its role in various applications:
- Chemical Classification: Propane is a hydrocarbon, more specifically an alkane, which means it consists only of hydrogen and carbon atoms with single bonds between the carbon atoms. Alkanes are also referred to as saturated hydrocarbons because they contain the maximum number of hydrogen atoms per carbon.
- Physical State: At room temperature and atmospheric pressure, propane is a gas. It is often compressed and stored as a liquid in cylinders and tanks for ease of transportation and storage, classifying it as a liquefied petroleum gas (LPG).
- Usage-Based Classification: In terms of its use, propane is commonly classified as a fuel. It is widely used for heating, cooking, and as a fuel for engines in both liquid and vapor form.
- Safety Classification: From a safety standpoint, propane is classified as a flammable and explosive gas, which means it poses risks of fire and explosion if not handled properly.
- Source Classification: Propane is considered a fossil fuel because it is derived from natural gas processing and petroleum refining. However, there are also efforts to produce renewable bio-propane from biomass.
- Environmental Classification: It is classified as a clean-burning fossil fuel since it produces fewer pollutants compared to coal or oil.
Understanding these various classifications is crucial for handling, storing, and utilizing propane safely and effectively while minimizing its environmental impact.
Propane in the Context of Organic Chemistry
A. Propane’s Place in the Alkanes Series
Propane is the third simplest member of the alkane series, a family of hydrocarbons with single bonds between carbon atoms. It follows methane (CH4) and ethane (C2H6) in the series and precedes butane (C4H10). Alkanes are characterized by the general formula CnH2n+2 and are known for their saturated nature, containing the maximum number of hydrogen atoms per carbon.
B. Chemical Reactivity of Propane with Other Substances
Propane is relatively inert due to the strength of its C-C and C-H bonds; however, it can still react under certain conditions:
- Combustion: Propane readily burns in the presence of oxygen, releasing energy, carbon dioxide, and water.
- Halogenation: In the presence of halogens and suitable conditions, propane undergoes substitution reactions, resulting in halogenated products.
- Cracking: When subjected to high temperatures and pressures, propane can break down into smaller hydrocarbons or alkenes, a process used in petrochemical industries.
C. Common Reactions Involving Propane and Their Industrial Significance
Propane’s reactivity is harnessed in various industrial applications:
- Fuel: As a combustible fuel, propane is used in heating, cooking, and auto gas.
- Petrochemical Feedstock: Propane can be cracked to produce propylene, an important feedstock for plastics and other chemicals.
- Refrigeration: Propane is used as a refrigerant in some systems, taking advantage of its thermodynamic properties.
Extraction and Synthesis of Propane
A. Natural Sources and Extraction Methods
Propane is primarily extracted from natural gas and oil. The process involves:
- Separation: Propane is separated from other hydrocarbons in natural gas or during the refining of crude oil.
- Fractional Distillation: It is often purified further by fractional distillation due to its higher boiling point compared to methane and ethane.
B. Synthesis of Propane in Industrial Settings
While most propane is extracted, it can also be synthesized in a laboratory setting:
- Alkylation: This involves combining smaller hydrocarbons like methane under specific conditions to form propane.
- By-product of Other Processes: Propane can also be obtained as a by-product in processes such as the cracking of larger hydrocarbons.
C. Sustainability and Environmental Considerations in Propane Production
The production and use of propane have environmental impacts that are being addressed through various measures:
- Efficiency Improvements: Improving the efficiency of propane extraction and usage to reduce emissions.
- Leak Mitigation: Efforts to minimize leaks during extraction, distribution, and storage to prevent loss and reduce the environmental impact.
- Alternative Sources: Research into renewable sources of propane, such as bio-propane, which is derived from biomass and offers a more sustainable profile.
What are the dangers of propane?
Propane, while widely used and generally safe when handled properly, does pose several dangers that are important to consider:
- Flammability and Explosive Potential: Propane is highly flammable. When mixed with air and exposed to an ignition source, it can ignite easily. If propane leaks, it can accumulate and create an explosive hazard. This risk is heightened in enclosed spaces where propane can build up.
- Health Risks from Inhalation: Inhaling propane can cause a variety of symptoms, including dizziness, headaches, nausea, and even asphyxiation at high concentrations due to the displacement of oxygen in the air.
- Risk of Frostbite: When propane is released from a compressed state, it can cause severe cold burns or frostbite, due to the rapid expansion and cooling effect.
- Combustion Byproducts: Incomplete combustion of propane can produce carbon monoxide (CO), a colorless, odorless gas that is toxic and can lead to CO poisoning. This typically happens with malfunctioning or improperly vented appliances.
- Environmental Concerns: Propane leaks can contribute to environmental pollution. Although it is not considered a direct greenhouse gas like methane or carbon dioxide, its presence in the atmosphere can have indirect effects on the environment.
- Storage and Handling Dangers: Propane tanks must be stored and handled properly to prevent leaks, ruptures, or accidents. Damage to tanks, improper refilling, or exposure to extreme heat can lead to dangerous situations.
- Odorless Nature: Propane is naturally odorless, which can make detecting leaks difficult. An odorant, typically ethanethiol, is added to give it a distinct smell and aid in leak detection.
- Pressure-Related Hazards: As a liquefied petroleum gas (LPG), propane is stored under pressure. If a propane tank is punctured or damaged, it can release gas rapidly, causing physical harm or increasing the risk of fire or explosion.
Awareness and adherence to safety guidelines, such as proper installation and maintenance of propane appliances, correct storage and handling of propane tanks, and the use of carbon monoxide detectors, can mitigate most of these risks.
Practical Applications of Propane’s Chemical Properties
A. Utilization in Fuel and Energy Generation
Propane’s high energy content and clean-burning properties make it an ideal fuel source in various settings:
- Home Heating: Propane is used in central heating, water heating, and cooking appliances in areas not served by natural gas pipelines.
- Agricultural Applications: It serves as a heat source for drying crops and heating animal enclosures.
- Power Generation: Propane-powered generators are used for backup power supplies and in remote locations.
- Transportation: Propane is used as an alternative fuel for buses, fleet vehicles, forklifts, and farm equipment due to lower emissions compared to gasoline or diesel.
B. Propane’s Role in Petrochemical Industries
Propane’s reactivity and availability make it a key raw material in the petrochemical industry:
- Production of Chemicals: It is a starting material for producing propylene and ethylene through steam cracking.
- Plastics Manufacturing: Propylene, derived from propane, is a precursor for polypropylene, a widely used plastic.
- Synthesis of Alcohols: Dehydrogenation of propane can produce isopropanol, a common solvent and disinfectant.
C. Specialty Uses in Organic Synthesis and Research
The unique properties of propane also find applications in scientific research and synthesis:
- Calibration Gas: Propane is used as a calibration standard in gas chromatography due to its consistent and well-defined properties.
- Refrigerant: In specific refrigeration systems, propane is used due to its low environmental impact compared to traditional refrigerants.
- Research Tool: Propane is used in studies of combustion, energy efficiency, and the development of new catalytic processes due to its well-understood combustion characteristics.
- Organic Synthesis: Although less common, propane can sometimes serve as a starting point for synthesizing complex organic compounds in a laboratory setting.
Health, Safety, and Environmental Impact
A. Propane’s Chemical Safety Profile
Propane, while useful, presents various health risks that necessitate careful handling:
- Asphyxiant: It can displace oxygen in the air, potentially leading to suffocation in enclosed spaces.
- Flammability: Highly flammable, posing fire and explosion risks if leaked in an enclosed area.
- Toxicity: Although not toxic when inhaled in small amounts, high concentrations can have narcotic effects.
- Cold Burns: Contact with liquid propane can result in severe cold burns or frostbite due to rapid expansion and cooling.
B. Handling and Storage Guidelines Based on Chemical Properties
Proper handling and storage practices are critical to mitigate the risks associated with propane:
- Cylinders and Tanks: Propane should be stored in well-ventilated areas, in tanks and cylinders designed to withstand its pressure.
- Leak Detection: Since propane is odorless, an odorant is added to ensure leaks can be detected. Regular leak checks are essential.
- Distance from Ignition Sources: Storage areas should be situated away from ignition sources due to the risk of explosion.
- Maintenance of Appliances: Regular maintenance of propane-burning appliances is important to prevent leaks and ensure complete combustion.
C. Environmental Implications of Propane Use and Leakage
Propane has a lesser environmental impact compared to other fossil fuels, but it still poses risks:
- Greenhouse Gas: While propane combustion produces less CO2 than other fossil fuels, it is still a contributor to greenhouse gas emissions.
- Volatile Organic Compounds (VOCs): Leaks of propane contribute to the formation of ground-level ozone, a component of smog, which can harm plant and animal life.
- Soil and Water Contamination: Liquid propane can cause freeze burns on plants and seep into groundwater, posing a contamination risk.
- Sustainability: Propane is a non-renewable resource, and its extraction and use are not sustainable in the long term. However, advances in bio-propane offer potential for a renewable alternative.
Conclusion
propane is unequivocally a chemical substance. It conforms to the definition by being composed of molecules that feature a specific arrangement of carbon and hydrogen atoms—three carbon atoms and eight hydrogen atoms (C3H8), to be exact. As a member of the alkane hydrocarbon series, it exhibits characteristics such as flammability, energy density, and reactivity that are foundational to its widespread use as a fuel and a petrochemical feedstock.
Despite its practical benefits, the handling of propane requires strict adherence to safety protocols due to its flammability, explosive potential, and the health risks posed by inhalation or direct contact. Environmentally, propane is a cleaner-burning alternative to other fossil fuels, but it is not without impact. Its role in contributing to greenhouse gas emissions and the potential effects on air quality and ecosystems from leaks are concerns that must be managed.
Propane’s status as a chemical is thus integral to understanding both its applications and the precautions necessary to ensure its safe and responsible use. As we continue to harness propane’s chemical properties for energy and industrial processes, ongoing research into mitigating its environmental impact and improving safety standards is essential.

Mike is an experienced propane technician with over 15 years of professional experience in the field. He has dedicated his career to helping customers with their propane needs, from installation to maintenance and repair. Together with Jeremy, he co-founded this website to provide useful information and guidance to customers seeking reliable propane services.







